A Psoriasis Commercial About Psoriasis Treatments Can Encourage
Watching a psoriasis commercial advertising psoriasis treatment plans is heartbreaking, especially when you have experienced the pain and swelling of psoriasis lesions and understand how hard it is to alleviate the symptoms. Advertisements promoting psoriasis medications usually involve only well-known and frequently prescribed drugs, such as Humira, Dovonex and Stelara, three drugs known for their successfulness in treating psoriasis plaques.
While a commercial may introduce you to certain psoriasis treatments, details about the medication are incomplete due to time constraints. The next time you view a psoriasis commercial, keep in mind it is only part of the whole story.
Ads About Humira
Humira is meant for grownups with modest to extreme psoriasis along with psoriatic joint inflammation that have trouble regulating episodes. Clinically described as a TNF, or lump death element medicine, Humira enhances the body's inflammatory feedback to neutralize psoriasis along with various other problems such as Crohn's illness, ankylosing spondylitis, rheumatoid joint inflammation and also some kinds of bronchial asthma.
While Humira benefits many psoriasis sufferers, it does carry the risk of side effects that are potentially serious. People taking Humira or any other TNF alpha-blocker should immediately report adverse conditions such as:
- Coughing, fever, rapid and unexplained weight loss as these symptoms may indicate a serious viral or bacterial infection.
- Extreme fatigue, yellowish skin and/or eyes, loss of appetite, fever, achiness that may be signs of Hepatitis B infection.
- Hives, breathing difficulties, facial swelling are classic symptoms of an allergic reaction.
- Vision problems, tingling and numbness in the arms and legs are indications of nervous system issues.
- Sudden and extreme weight gain, shortness of breath and feet/ankle swelling may be signs of impending heart failure.
In rare cases, Humira may cause worsening of psoriasis symptoms involving pus-filled bumps in addition to the usual scaly, painful lesions.
A Humira psoriasis commercial may not inform users that TNF blockers raise the risk of developing lymphoma and certain other cancers in both adults and children. I
nstances of a rare form of cancer called hepatosplenic T-cell lymphoma have occurred as a consequence of taking Humira in certain individuals sensitive to the effects of TNF blockers. Because Humira is injected, site irritations may occur that include redness, soreness and swelling.
A Psoriasis Advertisement About Dovonex
Dovonex is a calcipotriene ointment for topical use only that contains a synthetic derivative of vitamin D called calcipotriene. Side effects associated with Dovonex ointment are extremely rare, with allergic reactions being the most common. Initial burning and excessive dryness may occur but usually lessen as treatment continues.
Controlled trials with Dovonex have shown that after two weeks of using this medication, patients experience visible improvement with psoriasis lesions. After two months, nearly 60 percent of patients showed marked improvements, with 7 percent demonstrating complete remission of their psoriasis.
People using Dovonex should inform the physician prescribing the medication if they have hypercalcemia, or high vitamin D and/or calcium levels. Additionally, this ointment may make some individuals more sensitive to sunlight. Product instructions advise patients to avoid tanning beds and extended exposure to the sun.
A Psoriasis Commercial About Stelara
A psoriasis commercial about Stelara will reveal the following:
- Stelara treats psoriasis in people over 18 years old suffering from moderate to severe psoriasis.
- Research has not indicated that taking Stelara for more than two years is safe.
- Because it acts on the immune system, Stelara may increase the risk of developing cancer, serious infections and reversible posterior leukoencephalopathy syndrome, a rare condition affecting the brain.
- Patients who also suffer from a disease inhibiting protein production are more at risk for experiencing debilitating infections when taking Stelara.
- Women who breastfeed should not take Stelara, as this drug is passed into breast milk.
- Physicians give injections of Stelara in either 45 milligram or 90-milligram doses.
- Common side effects include headache and fatigue.
When watching a psoriasis commercial advertising medications not discussed here, pay attention to risks involved as well as method of application and any other warnings pertinent to your health situation. Also keep in mind, a healthier alternative natural treatment might be available as well.
Psoriasis commercial and other psoriasis medication
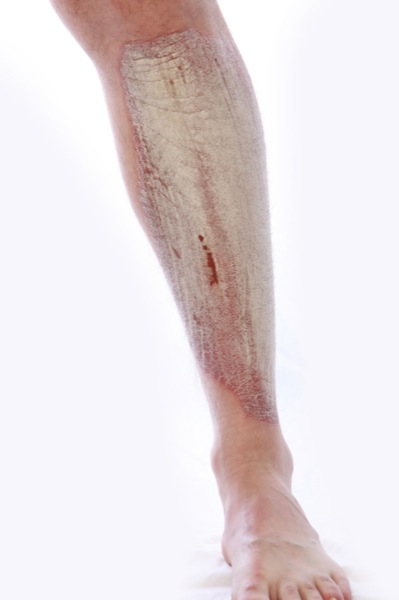 Psoriasis en la pierna
Psoriasis en la piernaPágina de inicio
Mapa del sitio
Mapa del sitio en orden alfabético
